P-V-T properties of a gas mixture describe how the pressure, volume, and temperature of a mixture of gases vary with the composition and the amount of the mixture. These properties depend on the behavior of the individual gases as well as their interactions with each other. There are two models that can be used to predict the P-V-T behavior of gas mixtures: Dalton’s law of additive pressures and Amagat’s law of additive volumes.
Dalton’s law states that the total pressure of a gas mixture is equal to the sum of the pressures that each gas would exert if it existed alone at the same temperature and volume as the mixture. The pressure of each gas in the mixture is called the partial pressure.
Amagat’s law states that the total volume of a gas mixture is equal to the sum of the volumes that each gas would occupy if it existed alone at the same temperature and pressure as the mixture. The volume of each gas in the mixture is called the component volume.
These models are valid for ideal gas mixtures, which assume that the gases do not interact with each other and occupy negligible volumes. For real gas mixtures, which deviate from the ideal gas behavior, more complex equations of state or compressibility factors are needed to account for the intermolecular forces and the finite volumes of the gas molecules.
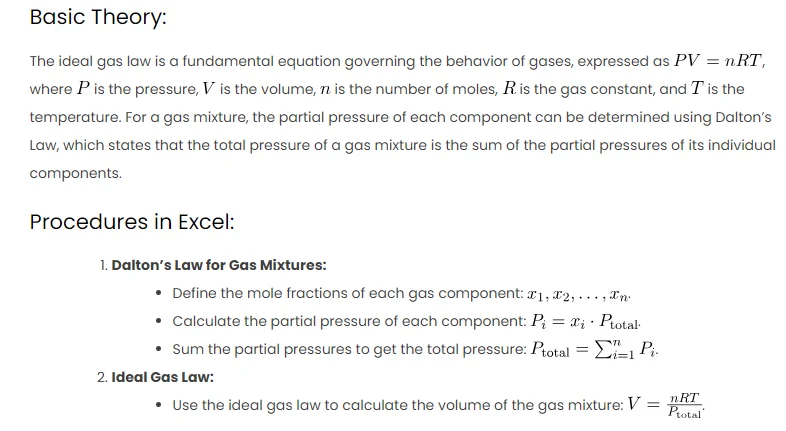
Basic Theory:
The ideal gas law is a fundamental equation governing the behavior of gases, expressed as , where
is the pressure,
is the volume,
is the number of moles,
is the gas constant, and
is the temperature. For a gas mixture, the partial pressure of each component can be determined using Dalton’s Law, which states that the total pressure of a gas mixture is the sum of the partial pressures of its individual components.
Procedures in Excel:
- Dalton’s Law for Gas Mixtures:
- Define the mole fractions of each gas component:
.
- Calculate the partial pressure of each component:
.
- Sum the partial pressures to get the total pressure:
.
- Define the mole fractions of each gas component:
- Ideal Gas Law:
- Use the ideal gas law to calculate the volume of the gas mixture:
.
- Use the ideal gas law to calculate the volume of the gas mixture:
Scenario:
Consider a gas mixture containing 40% oxygen (O2), 30% nitrogen (N2), and 30% carbon dioxide (CO2) at a total pressure of 5 atm and a temperature of 300 K.
Excel Calculation:
- Mole Fractions:
,
,
.
- Partial Pressures:
atm,
atm,
atm.
- Total Pressure:
.
- Volume Calculation:
.
Excel Table:
| Gas Component | Mole Fraction ( |
Partial Pressure ( |
|---|---|---|
| O2 | 0.40 | |
| N2 | 0.30 | |
| CO2 | 0.30 | |
| Total | 1.00 |
MATLAB Comparison:
For MATLAB, the calculations can be implemented using similar formulas. The script should include the definition of mole fractions, partial pressures, and the total pressure, followed by the volume calculation.
% MATLAB script
% Define mole fractions
x_O2 = 0.40;
x_N2 = 0.30;
x_CO2 = 0.30;
% Total pressure
P_total = 5; % atm
% Calculate partial pressures
P_O2 = x_O2 * P_total;
P_N2 = x_N2 * P_total;
P_CO2 = x_CO2 * P_total;
% Ideal gas law for volume
R = 0.0821; % atm·L/(mol·K) - gas constant
T = 300; % K - temperature
n_total = P_total * V / (R * T);
% Calculate volume
V = n_total * R * T / P_total;
Result:
Using the provided scenario and Excel calculations, the volume of the gas mixture is determined. This value can be compared with the MATLAB result to ensure consistency and accuracy in both approaches.