Estimation of ideal-gas heat capacity is the process of finding out how much heat is needed to change the temperature of a certain amount of an ideal gas by one degree. An ideal gas is a hypothetical gas that obeys the ideal gas law, which relates the pressure, volume, temperature and amount of gas. The heat capacity of an ideal gas depends on whether the gas is kept at constant volume or constant pressure, and on the number of degrees of freedom of the gas molecules. Degrees of freedom are the ways that the molecules can move or rotate. For example, a monatomic gas (such as helium) has only three degrees of freedom (translational), whereas a diatomic gas (such as oxygen) has five degrees of freedom (three translational and two rotational). The more degrees of freedom a gas has, the more ways it can store energy, and the higher its heat capacity.
The heat capacity at constant volume (CV) is the amount of heat needed to raise the temperature of a gas by one degree when the volume is fixed. In this case, the gas does no work, and all the heat goes into increasing the internal energy of the gas. The heat capacity at constant pressure (CP) is the amount of heat needed to raise the temperature of a gas by one degree when the pressure is fixed. In this case, the gas does some work by expanding, and some of the heat goes into doing this work. Therefore, CP is always greater than CV, because more heat is needed to raise the temperature of a gas at constant pressure than at constant volume.
The heat capacities of an ideal gas can be estimated by using the equipartition theorem, which states that each degree of freedom of a gas molecule contributes an equal amount of energy to the internal energy of the gas. The equipartition theorem can be used to derive the following formulas for the molar heat capacities of an ideal gas:
CV = (f/2)R
CP = (f/2 + 1)R
where f is the number of degrees of freedom, and R is the universal gas constant. These formulas are only valid for low to moderate temperatures, where the gas molecules do not vibrate or dissociate. At higher temperatures, the gas molecules may have more degrees of freedom, and the heat capacities may increase or decrease depending on the type of gas.
Basic Theory:
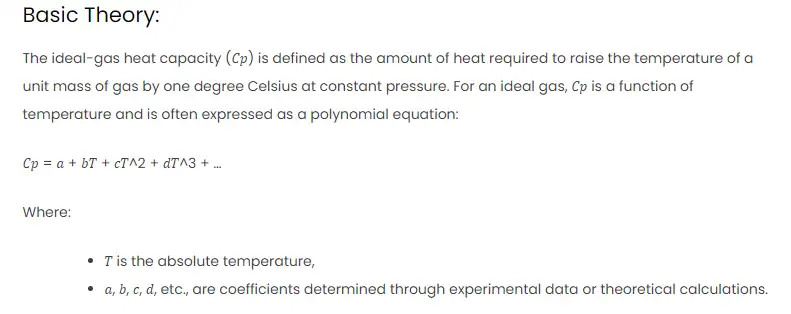
The ideal-gas heat capacity (𝐶𝑝) is defined as the amount of heat required to raise the temperature of a unit mass of gas by one degree Celsius at constant pressure. For an ideal gas, 𝐶𝑝 is a function of temperature and is often expressed as a polynomial equation:
𝐶𝑝 = 𝑎 + 𝑏𝑇 + 𝑐𝑇^2 + 𝑑𝑇^3 + …
Where:
- 𝑇 is the absolute temperature,
- 𝑎, 𝑏, 𝑐, 𝑑, etc., are coefficients determined through experimental data or theoretical calculations.
Procedures:
- Collect Coefficients: Obtain the coefficients 𝑎, 𝑏, 𝑐, etc., from reliable sources or conduct experiments to determine them.
- Create Excel Formula:
- Use the polynomial equation to create a formula in Excel, incorporating the coefficients.
- For example: 𝐶𝑝 = 𝑎 + 𝑏𝑇 + 𝑐𝑇^2.
- Input Temperature Values: Enter a range of temperature values into an Excel column.
- Apply Formula:
- In a neighboring column, apply the created formula to calculate 𝐶𝑝 for each temperature.
- Visualize Data:
- Create a table or chart to visualize the relationship between temperature and 𝐶𝑝.
Real-World Scenario:
Let’s consider a scenario with the following coefficients:
- 𝑎 = 20.5
- 𝑏 = 0.1
- 𝑐 = 0.002
Temperature values ranging from 100 K to 500 K will be used.
Excel Calculation:
| Temperature (K) | 𝐶𝑝 (J/mol-K) |
|---|---|
| 100 | =20.5+0.1*A2+0.002*A2^2 |
| 200 | =20.5+0.1*B2+0.002*B2^2 |
| 500 | =20.5+0.1*F2+0.002*F2^2 |
MATLAB Comparison:
For comparison, let’s perform the same calculation in MATLAB using the coefficients and temperature range.
% MATLAB code
a = 20.5;
b = 0.1;
c = 0.002;
% Temperature range
T = 100:100:500;
% Ideal-gas heat capacity calculation
Cp = a + b * T + c * T.^2;
Result:
Compare the results from Excel and MATLAB to ensure consistency. The table and chart will provide a visual representation of the ideal-gas heat capacity trend with temperature.