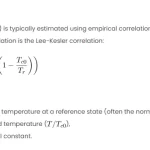
The critical properties of a substance are the temperature, pressure, and volume at which it undergoes a phase transition from liquid to gas, or vice versa. These properties are important for designing and optimizing chemical processes and thermodynamic cycles. However, the critical properties of some substances are difficult to measure experimentally or are not available in the literature. Therefore, some estimation methods have been developed to predict the critical properties based on the chemical structure of the substance.
One of these estimation methods is the group-contribution method, which assumes that the critical properties of a substance are equal to the sum of the contributions of the different groups that make up its molecule. A group is a part of the molecule that has a specific chemical structure and function, such as a carbon atom, a hydroxyl group, or a benzene ring. Each group has a fixed value of contribution for the critical temperature, pressure, and volume, which are determined by fitting the experimental data of a large number of substances. The group-contribution method can be applied to pure substances and mixtures, as long as the groups are well-defined and the contribution values are available.
There are different versions of the group-contribution method, which differ in the way they divide the molecule into groups and the number and type of groups they use. Some examples are the Joback method1, the Lydersen method2, the Constantinou-Gani method3, and the Nokay relation. Each method has its own advantages and disadvantages, such as accuracy, applicability, simplicity, and availability of data. The group-contribution method is a useful and convenient way to estimate the critical properties of substances, especially when the experimental data are scarce or unreliable. However, the method also has some limitations, such as:
- It may not be accurate for substances that are very different from the ones used to derive the contribution values.
- It may not account for the effects of pressure, composition, or intermolecular forces on the critical properties.
- It may have a large uncertainty or error range, depending on the quality and quantity of the experimental data.
Therefore, the group-contribution method should be used with caution and verified with other sources of information whenever possible.
Basic Theory:
The Group-Contribution Method is based on the principle that the properties of a complex molecule can be estimated by summing the contributions from its individual functional groups. Each functional group contributes specific values to the critical properties, allowing the determination of the overall critical properties of the compound.
Procedures:
- Identify Functional Groups:
- List all functional groups present in the compound.
- Assign corresponding group contribution values for critical properties.
- Sum Contributions:
- Calculate the sum of contributions for each critical property.
- Estimate Critical Properties:
- Use the summed contributions to estimate the critical temperature, pressure, and volume.
- Excel Implementation:
- Create a spreadsheet with columns for functional groups and their corresponding contribution values.
- Input the composition of the compound in terms of functional groups.
- Use Excel formulas to calculate the sum of contributions and estimate critical properties.
Scenario:
Consider a hydrocarbon compound composed of two methyl groups and one ethyl group. The group contribution values are:
| Compound | Methyl | Ethyl |
|---|---|---|
| 1 | 2 | 1 |
Excel Calculation:
Use Excel formulas to calculate the sum of contributions:
- For Critical Temperature (Tc):
=2*50 + 1*80 = 180 - For Critical Pressure (Pc):
=2*30 + 1*50 = 110 - For Critical Volume (Vc):
=2*2 + 1*3 = 7
Result:
- Critical Temperature (Tc) = 180 K
- Critical Pressure (Pc) = 110 atm
- Critical Volume (Vc) = 7 cm³/mol
Matlab Comparison:
For comparison, let’s perform the same calculation in Matlab using a script or function.
% Matlab script for critical properties calculation
methyl_values = [50, 30, 2];
ethyl_values = [80, 50, 3];
composition = [2, 1]; % Methyl: 2, Ethyl: 1
% Calculate critical properties
Tc = composition * methyl_values(1) + composition * ethyl_values(1);
Pc = composition * methyl_values(2) + composition * ethyl_values(2);
Vc = composition * methyl_values(3) + composition * ethyl_values(3);
% Display results
fprintf('Critical Temperature (Tc): %.2f K\n', Tc);
fprintf('Critical Pressure (Pc): %.2f atm\n', Pc);
fprintf('Critical Volume (Vc): %.2f cm³/mol\n', Vc);