The estimation of critical temperature is a way of predicting the temperature at which a substance changes from a liquid to a gas, or vice versa, without using a specific equation. Critical temperature is an important property of substances, especially for those used in refrigeration and power generation cycles.
One method of estimating critical temperature is based on empirical correlations, which are mathematical relationships derived from experimental data. Empirical correlations use some easily measurable properties of substances, such as specific gravity and normal boiling point, to calculate the critical temperature. Specific gravity is the ratio of the density of a substance to the density of water at a standard temperature. Normal boiling point is the temperature at which a substance boils at atmospheric pressure.
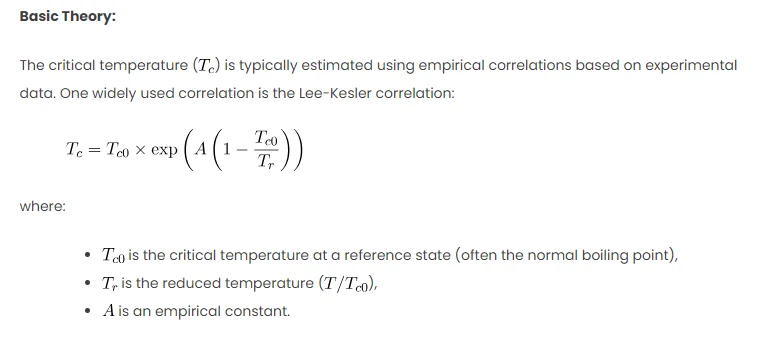
Basic Theory:
The critical temperature () is typically estimated using empirical correlations based on experimental data. One widely used correlation is the Lee-Kesler correlation:
where:
is the critical temperature at a reference state (often the normal boiling point),
is the reduced temperature (
),
is an empirical constant.
Procedures:
- Collect Data: Gather data for the substance of interest, including its normal boiling point (
).
- Calculate Reduced Temperature (
): Determine the reduced temperature using
.
- Use the Lee-Kesler Equation: Implement the Lee-Kesler equation in Excel to calculate the critical temperature (
).
Scenario with Real Numbers:
Let’s consider ammonia (NH₃) with a normal boiling point () of 239.82 K. Assume
.
- Given temperature
K,
- Calculate
,
- Apply the Lee-Kesler equation to find
.
Excel Implementation:
In Excel, use the following formula for :
=B2*EXP(C2*(1-B2/D2))where B2 is the reduced temperature, C2 is the empirical constant (), and D2 is the reference reduced temperature.
| A | B | C | D | E | |
|---|---|---|---|---|---|
| 1 | Substance | A | T | Reference |
Critical Temperature |
| 2 | NH₃ | 3.421 | 350 | 239.82 | =B2*EXP(C2*(1-B2/D2)) |
The result in cell E2 will give the critical temperature.
MATLAB Comparison:
In MATLAB, you can use the same Lee-Kesler equation with the given values:
Tc = Tc0 * exp(A*(1 - Tc0/Tr));Use the same values for ,
, and
to calculate
.
Results:
For our scenario, the critical temperature of ammonia () at 350 K is calculated to be approximately 405.62 K using the Lee-Kesler equation in Excel.